降煙鹼
| 降煙鹼 | |
|---|---|

| |
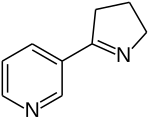
| IUPAC名 3-[(2S)-2-Pyrrolidinyl]pyridine | |
| 別名 | 去甲煙鹼 |
| 識別 | |
| CAS號 | 494-97-3 |
| PubChem | 91462 |
| ChemSpider | 82588 |
| SMILES |
|
| InChI |
|
| InChIKey | MYKUKUCHPMASKF-VIFPVBQEBM |
| 性質 | |
| 化學式 | C9H12N2 |
| 摩爾質量 | 148.2 g·mol−1 |
| 若非註明,所有數據均出自標準狀態(25 ℃,100 kPa)下。 | |
降煙鹼是存在於煙草等植物的生物鹼,化學式為C9H12N2。[1]它的結構與煙鹼相似,但少了一個甲基,因此又稱去甲煙鹼。
降煙鹼在醃製、加工煙草時會變為1類致癌物[2]N-亞硝基降煙鹼。[3]降煙鹼與口水反應也會產生N-亞硝基降煙鹼。[4]
合成[編輯]
藥學作用[編輯]
降煙鹼與煙鹼型乙酰膽鹼受體的親和力高[8],會以此抑制大鼠紋狀體的多巴胺轉運體,釋放多巴胺。[9][10][11]
參考資料[編輯]
- ^ Laszlo C, Kaminski K, Guan H, Fatarova M, Wei J, Bergounioux A, Schlage WK, Schorderet-Weber S, Guy PA, Ivanov NV, Lamottke K, Hoeng J. Fractionation and Extraction Optimization of Potentially Valuable Compounds and Their Profiling in Six Varieties of Two Nicotiana Species. Molecules. November 2022, 27 (22): 8105. PMC 9694777
 . PMID 36432206. doi:10.3390/molecules27228105
. PMID 36432206. doi:10.3390/molecules27228105  .
.
- ^ List of Classifications – IARC Monographs on the Identification of Carcinogenic Hazards to Humans. monographs.iarc.fr. [2020-07-22]. (原始內容存檔於2020-05-20).
- ^ Siminszky, B. Conversion of nicotine to nornicotine in Nicotiana tabacum is mediated by CYP82E4, a cytochrome P450 monooxygenase. Proceedings of the National Academy of Sciences. 2005, 102 (41): 14919–24. PMC 1253577
 . PMID 16192354. doi:10.1073/pnas.0506581102
. PMID 16192354. doi:10.1073/pnas.0506581102  .
.
- ^ Knezevich A, Muzic J, Hatsukami DK, Hecht SS, Stepanov I. Nornicotine nitrosation in saliva and its relation to endogenous synthesis of N'-nitrosonornicotine in humans. Nicotine & Tobacco Research. February 2013, 15 (2): 591–5. PMC 3611998
 . PMID 22923602. doi:10.1093/ntr/nts172.
. PMID 22923602. doi:10.1093/ntr/nts172.
- ^ Spaeth. Über dasd-Nor-nicotin. Chem. Ber. 1936, 69 (2): 250–251. doi:10.1002/cber.19360690207.
- ^ Haines. Chemical Reactivity of Myosmine. J. Am. Chem. Soc. 1945, 67 (8): 1258–1260. doi:10.1021/ja01224a011.
- ^ Dickerson, TJ; Janda, KD. Aqueous aldol catalysis by a nicotine metabolite. J. Am. Chem. Soc. 2002, 124 (13): 3220–1. PMID 11916401. doi:10.1021/ja017774f..
- ^ Papke RL, Dwoskin LP, Crooks PA. The pharmacological activity of icotine and nornicotine on nAChRs subtypes: relevance to nicotine dependence and drug discovery. Journal of Neurochemistry. April 2007, 101 (1): 160–7. PMID 17241116. doi:10.1111/j.1471-4159.2006.04355.x
 .
.
- ^ Middleton LS, Crooks PA, Wedlund PJ, Cass WA, Dwoskin LP. Nornicotine inhibition of dopamine transporter function in striatum via nicotinic receptor activation. Synapse. March 2007, 61 (3): 157–65. PMID 17146768. S2CID 35071082. doi:10.1002/syn.20351.
- ^ Dwoskin LP, Teng LH, Crooks PA. Nornicotine, a nicotine metabolite and tobacco alkaloid: desensitization of nicotinic receptor-stimulated dopamine release from rat striatum. European Journal of Pharmacology. September 2001, 428 (1): 69–79. PMID 11779039. doi:10.1016/s0014-2999(01)01283-3.
- ^ Dwoskin LP, Buxton ST, Jewell AL, Crooks PA. S(-)-nornicotine increases dopamine release in a calcium-dependent manner from superfused rat striatal slices. Journal of Neurochemistry. June 1993, 60 (6): 2167–74. PMID 8492124. S2CID 25622404. doi:10.1111/j.1471-4159.1993.tb03502.x.


![{\displaystyle \mathrm {\xrightarrow[{H_{2}O}]{Ag_{2}O}} }](https://wikimedia.org/api/rest_v1/media/math/render/svg/9e72a53e3814d3819989e4e6930a8e893b9c4225)

![{\displaystyle \mathrm {\xrightarrow[{H_{2}}]{Pd/C}} }](https://wikimedia.org/api/rest_v1/media/math/render/svg/efcf2036dece0161b4b1d5ffa6b0cbb27c449668)